Heart failure patients with an ejection fraction rate of greater than 40 percent can use Entresto (sacubitril/valsartan) regardless of the chronicity of the disease or treatment environment, a recent study has shown.
Dr. Robert Mentz of Duke University Medical Center unveiled the result of the PARAGLIDE-HF study, the phase 3 clinical trial of Entresto, at Heart Failure 2023, a scientific congress of the European Society of Cardiology (ESC) in Prague on Sunday (local time).
Global guidelines recommend the consideration of using sacubitril/valsartan to reduce hospitalizations in patients with heart failure with preserved EF (HFpEF; EF >50 percent) and/or mildly reduced EF (HFmrEF; EF 41-49 percent).
These were based on the PARAGON-HF study, where they compared Entresto and valsartan in 4,796 patients with an EF of 45 percent or higher. In the study, Entresto reduced total (initial or recurrent) heart failure hospitalization or death risk due to cardiovascular diseases by 13 percent compared to Valsartan.
Notably, it reduced such risks by 21 percent from valsartan in the subgroup of patients with EF of up to about 60 percent lower than normal.
“The PARAGON-HF trial excluded patients with decompensated heart failure, but a posthoc analysis suggested a larger benefit with sacubitril/valsartan in those recently hospitalized,” Dr. Mentgz said. “However, whether the initiation of sacubitril/valsartan is safe and effective in patients with EF over 40 percent-stabilized after a worsening heart failure event was unknown.”
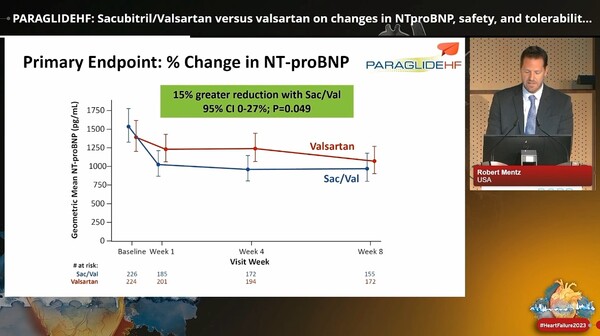
Accordingly, PARAGLIDE-HF evaluated the effect of sacubitril/valsartan versus valsartan on changes in NT-proBNP, safety, and tolerability in patients with EF above 40 percent who had been stabilized after a worsening heart failure event. The primary endpoint was the time-averaged proportional change in NT-proBNP from baseline at the fourth and eighth weeks.
As a result, the Entresto group showed a wider reduction in the time-averaged proportional change in NT-proBNP than the valsartan group (change ratio of 0.85 or 15 percent lower than valsartan).
The secondary composite hierarchical outcome consisted of time to cardiovascular death, number, and timing of heart failure hospitalizations, number and timing of urgent heart failure visits, and time-averaged proportional change in NT-proBNP from baseline to Weeks 4 and 8. The analysis results (like in each component) were advantageous for Entresto but were not statistically significant.
However, subgroup analyses showed evidence of a larger treatment effect in those with EF of 60 percent or more for the change in NT-proBNP (0.78; 95 percent) and the hierarchical outcome win ratio (1.46; 95 percent).
Regarding other secondary endpoints, sacubitril/valsartan reduced worsening renal function compared with valsartan, with an odds ratio (OR) of 0.61. There was also more symptomatic hypotension in the sacubitril/valsartan group (OR 1.73; 95 percent).
“PARAGLIDE-HF complements PARAGON-HF by focusing on patients stabilized after a worsening heart failure event with EF above 40 percent, similar to how PIONEER-HF complemented PARADIGM-HF in patients with reduced EF,” Dr. Mentz said.
“The latest data adds to evidence that supports potential treatment benefits provided by sacubitril/valsartan in patients with EF 40 percent or higher, especially those with lower-than-normal EF (less than 60 percent) patients,” Dr. Mentz said. “The result can affect guidelines for using drugs on acute, chronic or new heart failure patients in and outside hospitals.”
“PARAGLIDE-HF had no run-in period (the period between patient registration and random allocation), allowed both newly diagnosed heart failure and improved EF, included those with acute heart failure without specific echocardiographic requirements and overall had a diverse study population (52 percent women, 22 percent black individuals). The trial also permitted patients with eGFR down to 20 ml/min/1.73 m2, systolic blood pressure as low as 100 mmHg, and any BMI,” he said. “The broad and diverse population included in PARAGLIDE-HF supports the generalizability of these data to similar patients seen in routine practice.”
Meanwhile, a Korean expert drew attention, expressing his view that the study result can influence expanding reimbursement for Entresto in the domestic clinical environment.
“The hospitalization of heart failure patients with EF rate of 40 percent or higher is an issue to be resolved,” said Professor Cheong Wook-jin of the Cardiology Department at Gachon University Gil Medical Center. “There had long been no proper treatments for patients with EF exceeding 40 percent. Recently, sacubitril/valsartan and other new therapies received approval, but we still need additional clinical bases.”
He noted that sacubitril/valsartan had expanded its indications to heart failure patients with EF of 41-60 percent, but reimbursement has not expanded.
“The PARAGLIDE study supplements clinical ground for using sacubitril/valsartan for patients with EF of 41-60 percent who experienced acute aggravation recently, raising expectations about expanded reimbursement to this group of patients,” Professor Cheong said.

